Difference between revisions of "How to Heat Water with Sun Power"
(→Example) |
|||
| Line 47: | Line 47: | ||
</div> | </div> | ||
| − | It is more convenient for the examples that follow to measure energy in kWh. To convert megajoules (MJ) into kWh divide by 3.6 (1kWh = 3.6MJ) thus 16.8MJ = 4.7kWh. | + | It is more convenient for the examples that follow to measure energy in kWh. To convert megajoules (MJ) into kWh divide by 3.6 (1kWh = 3.6MJ) thus 16.8MJ = 4.7kWh. This is the amount of energy that must be put into the water. With many water-heating systems not all the energy used goes into heating the water, i.e. there are heat losses - the process is not 100% efficient. Some examples are given below. |
| − | + | Electric resistance heating is almost 100% efficient. Hence, to heat the water in the above example - 100 litres through a temperature rise of 40 <sup>o</sup>C - would require 4.7kWh of electricity, or for example a 1kW rated electric immersion heater running for nearly five hours. Although electricity is efficient at heating water it is expensive and not available everywhere. | |
| − | |||
| − | Electric resistance heating is almost 100% efficient. Hence, to heat the water in the above example - 100 litres through a temperature rise of 40 <sup>o</sup>C - would require 4.7kWh of electricity, or for example a 1kW rated electric immersion heater running for nearly five hours. | ||
| − | |||
| − | Although electricity is efficient at heating water it is expensive and not available everywhere. | ||
Water is usually heated by burning fuel. An oil (kerosene) or gas fuelled water heater has an efficiency of around 50%, while heating water on an open fire has an efficiency of about 10%. In this latter case to heat the 200 litres of water through 40 <sup>o</sup>C would require fire wood with a calorific value of nearly This is equivalent to about 10kg of low (15%) moisture wood 100kWh. | Water is usually heated by burning fuel. An oil (kerosene) or gas fuelled water heater has an efficiency of around 50%, while heating water on an open fire has an efficiency of about 10%. In this latter case to heat the 200 litres of water through 40 <sup>o</sup>C would require fire wood with a calorific value of nearly This is equivalent to about 10kg of low (15%) moisture wood 100kWh. | ||
Revision as of 14:29, 25 February 2012
Contents
Short Description
- Idea: Heat Water by Sun Power
- Difficulty: High
- Price Range: High
- Geographic Area: Temperate climate
Introduction
Hot water is required for many purposes and the sun can be used effectively, efficiently and economically to provide this heat. The warming effect of solar radiation is obvious and it is well known that a container of cold water, left exposed to the sun will be raised in temperature. Solar water heating systems are designed to make convenient use of this phenomenon.
Solar water heaters generally employ a solar collector and a storage tank. The solar water heating collector is by far the most widespread solar energy conversion device and there are several millions in use around the world. There are many simple designs of collectors and water heating systems. Construction and manufacture is easily achievable in most developing countries.
The energy required to raise the temperature of a substance is a physical property known as the specific heat of the particular substance. The specific heat of water is 4.2J/g/ oC, i.e. 4.2 joules of energy are required to raise the temperature of one gram of water by one degree centigrade. Using larger and more familiar units:
Energy required (kJ) = 4.2 x volume (litres) x temperature rise (oC)
Thus, in order to consider energy sources for water heating, the parameters that must be known are the volume of water required in a given time period (hour, day), the temperature of the 'cold' water, and the required delivery temperature. Hot water may be used for a variety of purposes, but as an example, domestic use is considerable. Here usage varies widely; in industrialised countries an average of around 50 litres per person per day is normal, in developing countries the more wealthy inhabitants may use this amount or more, while the poor may not use hot water at all.
Example
For the comparisons which follow a daily requirement for 100 litres of water at 60 oC with an ambient water temperature of 20 oC is used. Thus the energy requirement is:
|
4.2 x 100 x (60 - 20) |
= 16,800kJ |
|
= 16.8MJ |
It is more convenient for the examples that follow to measure energy in kWh. To convert megajoules (MJ) into kWh divide by 3.6 (1kWh = 3.6MJ) thus 16.8MJ = 4.7kWh. This is the amount of energy that must be put into the water. With many water-heating systems not all the energy used goes into heating the water, i.e. there are heat losses - the process is not 100% efficient. Some examples are given below.
Electric resistance heating is almost 100% efficient. Hence, to heat the water in the above example - 100 litres through a temperature rise of 40 oC - would require 4.7kWh of electricity, or for example a 1kW rated electric immersion heater running for nearly five hours. Although electricity is efficient at heating water it is expensive and not available everywhere.
Water is usually heated by burning fuel. An oil (kerosene) or gas fuelled water heater has an efficiency of around 50%, while heating water on an open fire has an efficiency of about 10%. In this latter case to heat the 200 litres of water through 40 oC would require fire wood with a calorific value of nearly This is equivalent to about 10kg of low (15%) moisture wood 100kWh.
By comparison, a simple solar water heater might have an efficiency of around 30%. On a very sunny day the solar energy received might be 6kWh/m2. Thus, to heat 100 litres of water through 40 oC would require a solar collector with an area of 4.7/(6 x 0.3) = 2.6m2.
In general:
The above example is intended only to give a rough indication. The energy available from the sun and the performance characteristics of solar collectors vary in a complex way and generalisations should be used with caution.
The availability of solar energy
The power density of solar energy reaches a maximum of about 1000W/m2 at sea level. This is made up of two components, the radiation in the direct beam from the sun, and diffuse radiation from the sky (radiation that has been scattered by the atmosphere). On a clear day diffuse energy may amount to 15-20% of the global irradiance whereas on a cloudy day it will be 100%.
Global irradiance varies throughout the course of the day because the path length of the solar radiation through the atmosphere changes. For the same reason, there are variations with season and latitude. The total solar energy received in a day (known as the insolation or solar irradiation) can vary from 0.5kWh m2/ in the UK winter to 5kWh/ m2 in the UK summer and can be as high as 7kWh/m2 in desert regions of the world. Many tropical regions do not have large seasonal variations and receive an average 6kWh/ m2/day throughout the year.
This variability is an important aspect of solar energy because it influences system design and solar energy economics. The size of the solar collector required for a particular application is dependent on the location under consideration.
The technology
When radiant energy strikes the surface of an object, a proportion (depending upon the angle of incidence and the nature of the surface) is reflected, part is absorbed and part may be transmitted through the object. With a few important exceptions, such as photovoltaic cells, the energy of the absorbed radiation is degraded rapidly to heat.
The balance between the absorbed input energy and the heat loss to the environment determines the temperature attained. The heat loss increases with the temperature and limits the ultimate temperature attained by a collector system. It also reduces the proportion of useful heat extractable from the system. Maximum temperatures and maximum useful power outputs are therefore obtained when a highly absorbent, well-insulated body is exposed to a high intensity of solar radiation. A wide range of systems, designed to meet a variety of needs and situations, have been developed and many are available commercially.
The best known solar heating device is the flat-plate collector, which is widely used for water heating in many parts of the world. The flat-plate collector absorbs as much as possible of the incident solar energy that falls upon it. Since the collector is normally fixed in position, the plate is close to perpendicular to the beam of sunlight (and therefore maximum absorption) for only part of the time, and the level of energy received therefore varies more strongly with time and season than does the actual intensity of the solar radiation. Because of the large areas over which heat can be lost, the retention of heat and hence the collection efficiency, falls off rapidly with increase in collection temperature. Since domestic water is normally needed at only about 50oC this is not normally a problem.
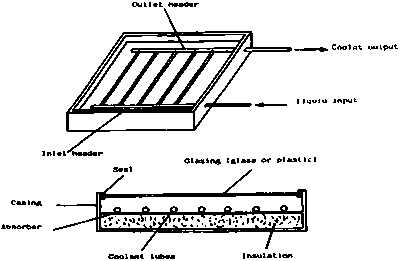
A simple flat-plate collector is shown in Figure 1. This consists of:
• an absorber that is painted black and from which heat is removed by a heat transfer fluid
• a cover which is transparent to solar radiation
• insulation at the back and sides of the absorber
• a casing to protect the absorber and its insulation.
The absorber may be made from one of a wide range of materials, including copper, stainless steel, galvanised steel, aluminium and plastics. When choosing an absorber material, it is important to ensure that it is compatible, from the point of view of corrosion, with the other components in the system and with the heat transfer fluid used. The absorber must also be able to withstand the highest temperature that it
might reach on a sunny day when no fluid is flowing in the collector (known as the stagnation temperature).
The fluid passageways of the absorber may consist of tubes bonded to an absorbing plate, or may form an integral part of the absorber.
Experience has shown that simple mechanical clamping of tubes to an absorber plate is likely to result in an absorber with a poor efficiency. A good thermal bond, such as a braze, weld or high temperature solder is required for tube and plate designs, in order to ensure good heat transfer from the absorbing surface into the fluid.
Matt black paints are commonly used for absorber surfaces because they are relatively cheap, simple to apply and may be easily repaired. Paints, however, have the disadvantage that they are usually strong emitters of thermal radiation (infrared), and at high temperature this results in significant heat losses from the front of the collector.
Heat losses from the collector can be substantially reduced by the use of absorber coatings known as 'selective surfaces'. These surfaces may be applied by electroplating or by dipping a metal absorber in appropriate chemicals to produce a thin semi-conducting film over the surface. The thin film will be transparent to solar radiation but at the same time appear opaque to thermal radiation. However, these surfaces cannot be produced or applied easily.
Flat-plate collectors usually have a transparent cover made of glass or plastic. The cover is required to reduce heat losses from the front of the collector and to protect the absorber and the insulation from the weather. Most covers behave like a greenhouse. They permit solar radiation to pass into the collector, but they absorb the thermal radiation emitted by the hot absorber.
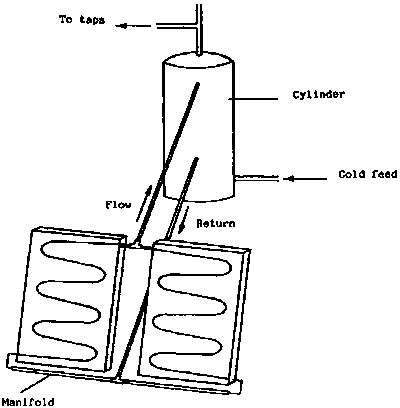
A solar water heating system consists essentially of a flat-plate collector and a water storage tank. The simplest arrangement is illustrated in Figure 2. In this, the tank is placed at a higher level than the collector so that the heated water will run from the collector to the tank and induce natural circulation by convection (thermosyphon). These systems can be very reliable provided the pipe-work diameter is adequate and there are not sharp bends. This is the most common design of solar water heaters used in developing countries.
At night it is possible for the collector to lose heat by radiation and the circulation will be in the opposite direction, so the water will cool. This can be overcome by use of a suitable non-return valve. However, there is a danger with solar collectors when used under clear night conditions (e.g. in arid and semi arid regions) that they can actually freeze even when the ambient temperature is above freezing point. In such conditions it may be necessary to have a primary circuit through the collector filled with antifreeze and a separate indirect hot water cylinder where the water from the collector passes through a copper coil to heat the main water supply. This problem will only apply in certain desert regions in the cold season or at high altitudes in the tropics and sub-tropics.
It is also possible to pump the water between the collector and tank. This allows the two components to be more widely separated and the tank does not have to be at a level higher than the collector, however, these systems are much more complex and electricity is required to power the circulating pump.
Costs
Flat-plate solar collectors typically cost £150 per square metre and a professionally installed system costs around £2,000 or more. In the UK, the economics are marginal because savings on fuel will be approximately £100 a year. In sunnier locations, however, payback periods of just a few years are possible. The use of solar water heating displaces the burning of other fuels and hence is beneficial to the environment.
References and further reading
This Howtopedia entry was derived from the Practical Action Technical Brief Solar Water Heating - Technical Brief .
To look at the original document follow this link:
http://www.practicalaction.org/?id=technical_briefs_water
The following publications provide more detailed, easy to understand, information on solar water heating.
• Solar Water Heating: A D-I-Y Guide CAT Publications - 1999
• Heating Water by the Sun UK-ISES 1981
• Solar Domestic Hot Water by Plante R H Wiley and Sons - 1983
• Practical Solar Heating McCarthy K/Ford B - - Prism Press - 1978
James and James produce quite a few renewable energy publications including; The World Directory of Renewable Energy, Renewable Energy World, and Solar Energy Houses.
Usefull addresses
Practical Action
The Schumacher Centre for Technology & Development, Bourton on Dunsmore, RUGBY, CV23 9QZ, United Kingdom.
Tel.: +44 (0) 1926 634400, Fax: +44 (0) 1926 634401
e-mail:practicalaction@practicalaction.org.uk web:www.practicalaction.org

James & James (Science Publishers) Ltd.
35 - 37 William Road, London, NW1 3ER, United Kingdom
Tel: +44 (0)20 7387 8558, Fax: +44 (0)20 387 8998
Website: http://www.jxj.com
Information can also be obtained from the following organisations:
The Solar Energy Society
School of Engineering, Oxford Brooks University, Gipsy Lane Campus, Headington, Oxford, OX3 0BP, United Kingdom
Tel: +44 (0)1865 484367, Fax: +44 (0)1865 484263
Website: http://www.brookes.ac.uk/uk-ises
The Solar Energy Society is a non-profit organisation. It is a forum for all those interested in the advancement of the utilisation of the sun's energy. Members of the Society are drawn from industry, government, academic institutions, architectural and engineering practices, as well as the general public: academic qualifications are not a pre-requisite for membership.
NEF Renewables,
The National Energy Foundation, Davy Avenue, Knowlhill, Milton Keynes, MK5 8NG, United Kingdom
Tel: +44 (0)1908 665555, Fax: +44 (0)1908 665577
The Solar Trade Association Ltd*
Pengillan, Lerryn, Lostwithiel, Cornwall, PL22 0QE, United Kingdom
Tel: +44 1208 873518, Fax: +44 1208 873518
Website: http://www.greenenergy.org.uk/sta/
Trade association for the UK. Manufactures and installers of solar equipment.
The Information Officer *
Solar Energy Unit, University College of Wales, Cardiff, Newport Road, Cardiff, CF2 1TA, United Kingdom
*Can supply a list of solar collector manufacturers.
Categories
- More than 200 US$
- Community
- Construction
- Energy
- Equipment Design
- Health
- Heating
- Ideas
- Mechanics
- Resource Management
- Sanitation
- Small Business
- Water
- Sun
- Medium
- Global Technology
- Global
- Mediterranean Climate
- Monsoon Climate
- Montaneous Environment
- Coastal Area
- Rural Environment
- Temperate Climate
- Tropical Climate
- Urban Environment
- One Person and more
- Household
- Village
- Neighbourhood
- Application
- Principles
- Requested translation to Spanish