How to Store Electricity in Batteries
Contents
Short Description
- Problem:
- Idea:
- Information Type: Application / Principles
- Difficulty:
- Price Range:
- Material Needeed:
- Geographic Area:
- Competencies:
- How Many people?
- How Long does it take?
Batteries
As many small-scale methods of electricity generation are available only intermittently, some form of electricity storage or battery is needed if people want to have electricity available at all times.
There are a wide range of batteries available, and the aim of this Technical Brief is to give an introduction to the advantages and disadvantages of the different types of batteries. The central point is that there is no such thing as a universal battery; a single type of battery cannot cover all applications. You can find a more in-depth description of how batteries work, the terms and definitions used to specify rechargeable batteries, and details about charging battery systems in Chapter 7 of Rural Lighting, ITDG Publishing.
|
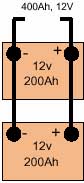
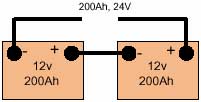
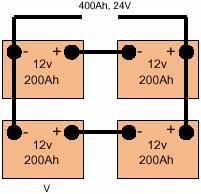
Cell It is normal to connect cells in series so their voltage adds up to the required value. For example, 6V can be achieved by connecting in series three 2.0V lead-acid cells or five 1.2V nickel-cadmium cells Battery A packaged combination of cells is technically known as a 'battery'. In most cases a number of cells packaged in a single container or sleeve, typically three or six 2V lead-acid cells to give a 6 or 12V battery. Series connection If cells or batteries are connected + to - (i.e. positive of one cell to negative of the following cell) so that their voltage add to a suitable value for the application, they are said to be 'series connected'. All cells will have the same current passing through them. Parallel connection If two or more cells or batteries are connected + to + and - to - (i.e. positive of one cell to the positive of another and similarly for the negative poles) then they are said to be parallel connected'. Two cells connected in parallel would produce the same voltage as a single cell, but be capable of delivering twice the current. They would also have twice the electrical storage capacity of a single cell at the same voltage. |
Figure 1: Cell, battery and connection definitions
Batteries can be sub-divided into the following types:
Primary cells or dry batteries
- standard zinc-carbon
- alkaline or heavy duty
Secondary cells or rechargeable batteries
- Lead-acid battery
- vented lead-acid
- automotive (car)
- deep-discharge or traction
*stationary
- low-antimony solar battery
- sealed or valve-regulated
Nickel- Cadmium batteries
- vented
- sealed
Primary cells - Dry batteries
The familiar flashlight battery is perhaps the most commonly used battery, particularly in the South. This type of battery comes in standard sizes of AAA, AA, C, and D.
Although the purchase or first cost of dry cells is relatively low, it is one of the least cost-effective electrical power sources in terms of the cost per unit of useful energy delivered. Furthermore, only a limited energy yield can be obtained before the battery has to be thrown away. Dry batteries are used in especially large numbers by the poor, as they are convenient, just about affordable, and generally all that is available. Their high cost makes them only suitable for powering small appliances that can only be used economically for short periods or emergencies.
Primary cells are based on an irreversible electrochemical reaction, and consequently cannot be recharged. Once the chemicals inside the battery are exhausted the battery is useless and must be disposed of. In recent years primary cell technology has improved dramatically, and two distinct qualities of cell are usually available in any size: standard zinc-carbon, and alkaline (also called 'heavy duty' or 'long life').
Zinc-carbon cell
The most widely used and cheapest form of primary cell, especially in the South, is the zinc-carbon cell. The voltage of any zinc-carbon cell is 1.3 to 1.5 volts when the chemicals are fresh. The size of the cell only influences the current (and hence the power) that can be produced.
Alkaline cells
Alkaline cells are more sophisticated in design than zinc-carbon cells, and have a much larger electrical capacity. Alkaline cells are also called manganese dioxide cells, or 'heavy duty'; or 'long life' batteries. Their open voltage is 1.5 volts when the chemicals are fresh.
How it works
As a cell discharges its voltage falls. A fresh zinc-carbon cell may have an open voltage of 1.5V, for example, but towards the end of its useful life the voltage will fall to around 0.8 to 0.9V.
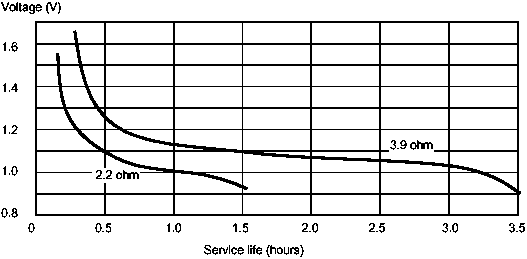
Figure 2: Continuous rate of discharge of a typical zinc-carbon C-sized cell
The electrical capacity of a cell is the total quantity of electricity that a cell can deliver. The potential electrical capacity of fresh cells of the same size and type is the same, but the true capacity is not fixed, it depends on many factors, such as cell size, cell type, rate of discharge, temperature, and mode of use. For a given type, the bigger the cell, the higher the electrical capacity. The electrical capacity of the cell is used up at a much greater rate the higher the current. Flashlights, for example draw 0.3 to 0.5A from a D cell by 50 per cent. If used continuously, the situation is even worse; the D cell may deliver only 25 per cent of its rated capacity.
In order to optimise the use of dry cells, it is a common practice to use them in radios and cassette players until their voltage falls (most electronic devices need a minimum voltage to function at all), and then the cells are finished off' in flash-lights, where a battery with low voltage simply results in a rather dim and yellow light.
Factors affecting useful life
The capacity of dry cells, like most other batteries, increases at higher temperatures. The capacity is usually given at 20°C; above this temperature the capacity is increased, and below this temperature capacity is decreased, so warming the batteries before use will result in extra power.
Primary cells are stable in terms of self-discharge. Some of the alkaline 'heavy duty' types can be kept for several years with no more than a few per cent loss of capacity.
|
Characteristics of primary cells compared with miniature secondary cells | ||||||
|
Type of cell |
Size of cell |
No. of cycles |
Nominal capacity |
Useful1 Energy |
Cell cost |
Unit2 cost |
|
ah |
Wh |
US$ |
US$/kWh | |||
|
Zn-C |
D |
1 |
5.5 |
2.2 |
1.0 |
450.0 |
|
Alkaline |
D |
1 |
16.0 |
14.0 |
2.0 |
140.0 |
|
Ni-cad |
D |
100 |
4.0 |
400.0 |
8.5 |
21.0 |
|
D |
200 |
4.0 |
800.0 |
8.5 |
10.6 | |
|
D |
500 |
4.0 |
2000.0 |
8.5 |
4.25 | |
|
1. Useful energy (Wh) has been obtained from typical characteristic curves of dry cells and for a load typical for a small lighting application (e.g. a flashlight: discharge rate 0.5A two cells in series for a 1.2W/2.5V bulb). 2. The unit cost indicates the cost of the battery per unit of output. A rechargeable battery also needs a charging source which adds to the cost by a variable amount depending on the type of charger. Obviously the charger will generally last many more cycles than an individual ni-cad cell. Examples of 100, 200, and 500 cycles for the ni-cad cells are given. | ||||||
The cheaper zinc-carbon type deteriorate more quickly, but even so they retain their capacity better than any other type of portable electrical power source. The self-discharge rate is adversely affected by high temperature, so store the cells at between 10 and 25°C and at a relative humidity of below 65 per cent.
Cost
The cost of electricity from primary cells varies widely between US$140 and $1300 per kWh, and is about 700 to 6500 times more expensive than mains electricity taken at $0.2 per kWh. The initial cost of primary cells is low, but the unit cost of electricity from them is extremely high. Despite this, the use of primary cells remains common, partly because the cost is spread over a period of time, partly because they are convenient, but mainly because they are often the only source of power available, particularly in rural areas.
Secondary cells: Rechargeable cells and batteries
There are two main types of secondary cell in general use: lead-acid and nickel-cadmium (NiCd).
Nickel-cadmium batteries
The main alternative to the lead-acid battery is the nickel-cadmium or 'ni-cad' battery. Like lead-acid, ni-cad batteries are available either vented or sealed. Vented ni-cad are designed for applications which require robust energy storage with long operating lifetimes and minimal maintenance. Sealed and usually small (i.e. sized AAA, AA, (, or D), ni-cad batteries are used as an economical replacement for dry cells.
The nominal voltage of a ni-cad cell is 1.2 volts, so a nominal 12V ni-cad system needs 10 cells. Ni-cad cells can withstand a greater depth of discharge than lead-acid batteries, and so generally a smaller capacity can serve a given duty. They also tend to last longer, 10 to 20 years for the larger ones. Ni-cads are less easily damaged by over-discharge or overcharging, and so simpler and cheaper charge control systems can be used to compensate for their extra unit costs. They are also more tolerant of extreme temperature variation than lead-acid batteries, and can operate at sub-zero temperatures.
Although ni-cad batteries are robust and reliable, they do have a few shortcomings that can cause problems. One major problem is that reversing the polarity when recharging a ni-cad cell usually destroys it completely. This can sometimes happen, not because a cell was reversed by carelessness when wiring it up for recharging, but when one cell in a battery of ni-cad cells is weaker than the rest: then the good cells can cause reverse charging of a weak one in certain circumstances, destroying the weak one completely. This is one reason why it is not a good policy to mix old cells and new ones either for recharging or for actual use.
|
Principal characteristics of various batteries | ||||||
|
Type |
Depth of discharge |
Self-discharge (capacity per month) |
No. of cycles |
Calendar life (cell life) |
Approx cost |
Approx. cost |
|
% |
% |
years |
US$/kWh |
US$/kWh | ||
|
Lead acid |
||||||
|
Automotive |
20 |
30 |
300-600 |
1-3 |
100-150 |
80 |
|
80 |
20 |
|||||
|
Traction |
80 |
5-7 |
1500 |
4-6 |
200-400 |
200 |
|
Stationary |
50 |
3 |
3000 |
5-10 |
300-4000 |
250 |
|
80 |
1200 |
|||||
|
Solar |
50 |
1-3 |
3000 |
5-10 |
250-350 |
200 |
|
Low antimony |
80 |
3 |
1200 |
|||
|
Sealed |
20 |
2-6 |
400-1500 |
4-8 |
150-500 |
200 |
|
Ni-cad |
||||||
|
Sealed |
100 |
5-30 |
100-10000 |
3-5 |
600-1000 |
N/A |
|
Unsealed |
100 |
3-5 |
1000-2000 |
20 |
5000 |
350 |
|
Note: the cost in US$/kWh is calculated as follows: price of battery divided by rated capacity. | ||||||
Another characteristic of ni-cad batteries is a tendency to self-discharge rather more quickly than lead-acid cells and much more quickly than primary cells. Ni-cad primary cell substitutes therefore need regular recharging and are less useful for occasionally used loads than for regularly used ones. They are particularly well suited for small photovoltaic application where they are being charged with daily sunshine.
Memory effect of ni-cad batteries
The memory effect is the tendency of a battery to adjust 'its electrical properties to a certain duty cycle to which it has been subjected for an extended period of time. Vented pocket-plate batteries do not develop this effect, but sealed cells, such as the AAA, AA, C, and D sizes do. To remedy this problem, they need to be 'awakened' by being fully charged and discharged for three or four cycles before their memory is 'stretched' enough to hold a full charge.
Costs
The small ni-cad batteries have a higher initial cost than a primary cell, but work out much less expensive in the long run since they can be recharged and re-used from 100 to 1000 times before they lose their capacity and need to be replaced. Obviously, a suitable power source is necessary to recharge them, which could be a special low-voltage charger powered by the mains or a generating set, or by solar photovoltaics. Large nickel-cadmium batteries can also be financially competitive with large (over 100Ah) lead-acid batteries, bearing in mind that they can be 100 per cent discharged while a lead-acid battery generally should be limited to 50 to 70 per cent discharge of its rated capacity.
Lead-acid
The least expensive option for any significant size of electrical battery storage is the lead-acid battery. Lead-acid batteries have a nominal fully charged voltage of 2V per cell, so a 12V battery typically has six cells in series. A lead-acid battery will only withstand a certain number of charge-discharge cycles, before it fails and needs to be re-placed. The greater the depth of discharge (that is the more on average that the battery is 'flattened'}, the fewer cycles it will survive. For example a battery that is discharged regularly by 80 per cent of its total capacity may last 800 cycles, but if it is discharged by only 20 per cent each time it may last 6000 cycles. If the battery were discharged at 20 per cent rather than 80 per cent, the rated capacity will have to be four times larger to deliver the same energy, but will last at least four times as long. The size of the battery is therefore a compromise between making it large but too expensive, and small and affordable but too easily discharged and therefore too short-lived.
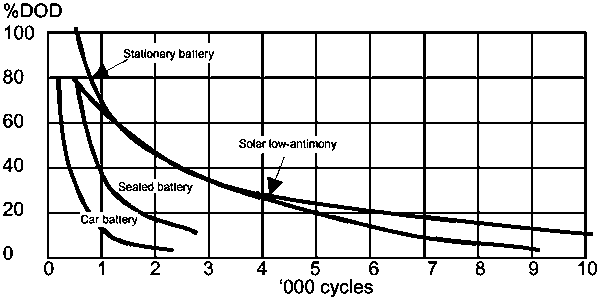
Figure 3: Cycle life verses depth of charge for several types of lead-acid battery
A lead-acid battery's capacities are usually specified for 25°C operating temperature. The capacity is typically reduced by 1 per cent per 1°C going down to 0°C, but increases approximately 1 per cent per 1°C, going up from 25°C to 40°C. The problem is that the life of the battery decreases with increased temperature so, in a tropical climate, a battery should be kept whenever possible in a cool and well ventilated room.
As many small-scale methods of electricity generation are available only intermittently, some form of electricity storage or battery is needed if people want to have electricity available at all times. Lead-acid batteries can be simply sub-divided into five categories, the first four of which are vented:
- Automotive
- Deep-discharge or traction
- Stationary
- Low-antimony solar battery
- Sealed or valve-regulated battery
Automotive batteries
Automotive batteries have a poor capacity for their size and a poor cycle life. A typical automotive battery will only withstand about 20 deep-discharge cycles before it becomes completely useless. Car batteries are also easily damaged if left discharged for any length of time. The cell design in a car battery is optimised to deliver heavy currents, and it is therefore poorly suited to supplying smaller currents for many hours before being recharged.
Car batteries are, however, usually the cheapest batteries when compared by rated capacity; they are often produced locally; and they are widely available and repairable.
Deep discharge or traction batteries
Deep-discharge batteries can tolerate discharge to as much as 80 per cent of their rated capacity, with a cycle life of from 1000 to 1500 deep cycles. They tend to lose water at a faster rate than other types of lead-acid battery, and need frequent maintenance. They are commonly used for electric' vehicles and are often known as traction batteries. Their self-discharge rate is also high. These batteries are relatively expensive, require a lot of maintenance, and are not often available locally.
Stationary batteries
These batteries are often called stand-alone or standby batteries, and have been designed to supply power when there is a grid failure. In most applications they are kept fully charged by the mains supply and are ready to take the load whenever needed. They are extremely reliable, have a low self-discharge rate, and a long cycle life with shallow cycles, lasting up to ten years. These batteries are usually oversized when used for stand-alone applications, to ensure that they only run with shallow cycles and last a long time.
Low-antimony solar batteries
These batteries are similar to stationary ones, but have been designed for photovoltaic systems. The self-discharge rate and distilled water consumption are both low. The cycle ranges from 1200 to 3000 depending on the discharge rates. These batteries are fairly expensive and available only run with photovoltaic systems suppliers.
Sealed or valve-regulated batteries
The hydrogen produced by these batteries is absorbed by chemicals inside them and they contain enough electrolyte for their entire life, so they are often called 'maintenance-free'. Sealed batteries have a short cycle life for deep cycles. They have a low rate of self-discharge and can support a full discharge, but must be recharged as soon as possible to prevent permanent damage.
Overall, a sealed battery is likely to have a shorter life than a well-maintained unsealed battery with the same alloy contents, but will obviously last longer than a poorly maintained unsealed battery.
The main disadvantage of sealed lead-acid batteries is their need for regular recharging to prevent sulphate build-up. Batteries in storage will need to be recharged about once every three months, more often in countries with high ambient temperatures where self-discharge will happen more quickly.
|
Safety and environmental hazards of lead-acid batteries Vented Batteries: Care is obviously needed as, part from the battery acid being extremely corrosive, hydrogen gas is produced, which is highly flammable and potentially explosive when mixed with air. Thus care should also be taken to avoid naked flames or sparks in the battery enclosure, especially if the battery is housed in a confined space. Never check the electrolyte levels with a naked flame such as a kerosene lamp or a candle. For the same reason, battery storage areas should be well ventilated Sealed Batteries: These contain the electrolyte in 'dry' from so that no electolyte can be spilt, and so there is less of a hazard. Even so, care must be taken not to damage the casing. Recycling: Both types of batteries should be deposed of safely. Where practical, it is a good idea to give away lead-acid batteries to local battery manufactures for lead and plastic-casing recycling. Ni-cad batteries should be disposed of carefully to avoid cadmium pollution |
References and Further Reading
- Rural Lighting: A Guide for Development Workers Jean-Paul Louineau, Modibo Dicko, Peter Fraenkel, Roy Barlow and Varis Bokalders, ISBN 1 85339 200 6, ITDG Publishing, 1994.
- Batter Charging in Colombia MHPG Mini Hydro Facts
- Fuel Cells by Teodoro Sanchez Practical Action Latin America
|
This technical brief was originally written for the Appropriate Technology magazine Volume 21/Number 2 September 1994 ATBrief No 9, For more information about Appropriate Technology contact: |
References and further reading
This Howtopedia entry was derived from the Practical Action Technical Brief Energy from the Wind.
To look at the original document follow this link: http://www.practicalaction.org/?id=technical_briefs_energy
Useful addresses
Practical Action
The Schumacher Centre for Technology & Development, Bourton on Dunsmore, RUGBY, CV23 9QZ, United Kingdom.
Tel.: +44 (0) 1926 634400, Fax: +44 (0) 1926 634401
e-mail: practicalaction@practicalaction.org.uk
web: www.practicalaction.org

Related articles
- How to Provide Lighting in Rural Areas-Principles-
- How to Build a Windpump (Principles)
- How to Use Energy from the Wind
- How to Plan a Micro Hydro-power Plant
- How to Build a "Water Motor"
- How to Build a Small Wind Turbine
- How to Generate Wind Electricity (Principles)
- How to Use Energy from the Wind
- How to Provide Electricity in Rural Areas-Principles-
- How to Build Small Educational Wind Turbine
- How to Store Electricity in Batteries
Two ways to support the work of howtopedia for more practical articles on simple technologies:
Support us financially or,
Testimonials on how you use howtopedia are just as precious: So write us !
<paypal />